Carbon Valence, Hybridization and Angles Virtual Lab
Join Dr. One on a mission to figure out how the orbitals of carbon’s valence electrons hybridize, and how this greatly influences the bonds that carbon is able to form.
Try our lab safety simulation
Discover one of our 200+ learning simulations today
- Description
- Features
关于碳价,杂交和角度Virtual Lab
This simulation was adapted from the original, longer “Organic Chemistry Introduction” simulation. See the full-length simulation for more information.
Get your hybridization right! In this simulation, you will learn why the element carbon forms four chemical bonds to be in a stable state. You will explore the involved valence electrons and their orbitals, and how hybridization is key to forming equal, stable bonds.
Organic chemical compounds
You will start off by meeting with Simon, who’s having trouble figuring out the ins and outs of the fundamentals within organic chemistry. Try to resolve his confusion about what, exactly, is meant by ‘organic’. Perhaps you will be able to sort him out?
Dive into the orbitals
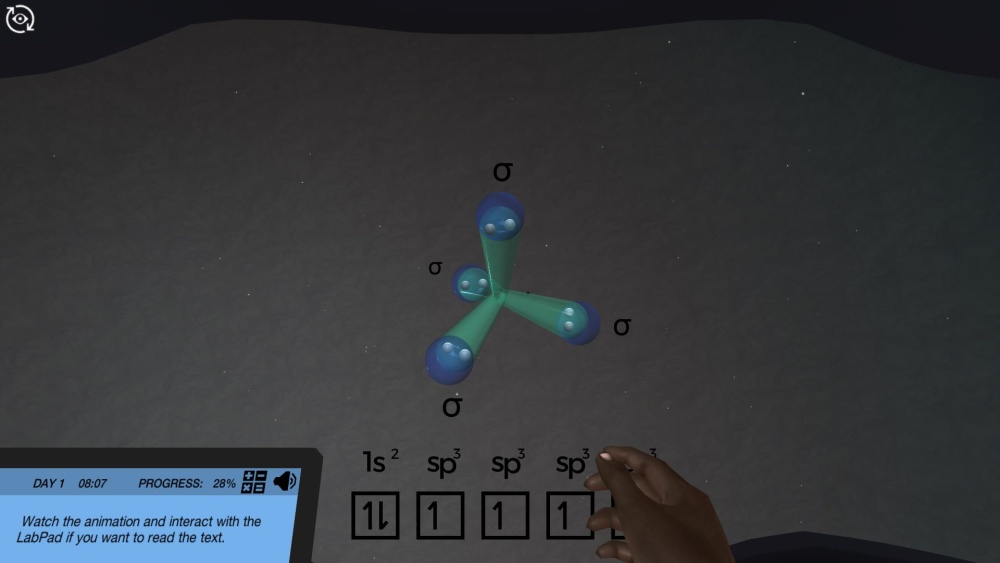
In the lab, you join forces with Dr. One, who will support and test you on your mission. After getting suited up for being in the lab, and getting a quick briefing on organic compounds, you jump into a virtual animation on the valence and orbitals of carbon. You’ll need to pay close attention, as Dr. One will quiz you afterwards! Should you be in trouble, you can always jump over to the theory pages for help in finding the correct answer.
Getting the bond angles right
Now that you’re prepped with the fundamental knowledge of carbon bonds, it will be time to put it all into action. Move on to the holotable, where you will be challenged with figuring out the placement of hydrogens in common organic compounds, factoring in double and triple bonds.
Returning to Simon
Armed with all your new knowledge, you will return to Simon to share all you’ve learnt. It’s a tricky topic though, but luckily you can repeat the experience if you want to drive the points in even further. Will you be able to solve the carbon hybridization challenge?
RELATED SIMULATIONS
Organic Chemistry Introduction: Learn about organic compounds
Hydrocarbon Nomenclature and Representations
Functional Groups and Basic Chemical Tests
Join Dr. One on a mission to figure out how the orbitals of carbon’s valence electrons hybridize, and how this greatly influences the bonds that carbon is able to form.
At the end of this simulation, you will be able to...
- Give examples of uses of organic compounds
- Identify the carbon valence electrons and the hybridization of their orbitals
- Predict the angles of covalent bonds in hydrocarbons
Length: 25 Minutes
Accessibility mode: Not available
Languages: English (United States), Spanish, Italian, German, French
HS-PS1-3, HS-PS1-6
10.1 Fundamentals of organic chemistry
10.2 Functional group chemistry
20.1 Types of organic reactions
Unit 2: Ionic Compounds Structure and Properties