In 1937, Robert Hill discovered that isolated chloroplasts can produce oxygen even if no carbon dioxide is present. The only requirements to convert water to molecular oxygen are an electron acceptor and light.
We can use a special electron acceptor called aredox染料来衡量的电子流electron transport chain. DCPIP is a redox dye. In its oxidized state it absorbs light in the red spectrum and it appears dark blue. In its reduced state however it does not absorb light of the visible spectrum and is, therefore, colorless.
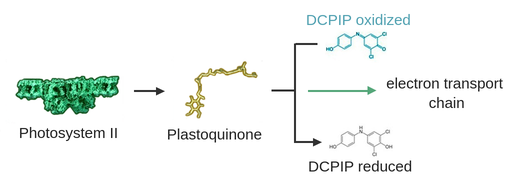
These properties in combination with its ability to diffuse into biological membranes make it the ideal indicator to measure the redox potential of the electron transport chain. So, we can measure the electron flow from photosystem II.
来衡量the activity of the photosystems, the cells should be kept in the dark for a day before starting with the experiment. Dark incubation ensures that all the components of the electron transport chain are in their lowest energy state.
DCPIP inside the thylakoid membrane gets reduced by the plastoquinone that would naturally transfer the electrons onto the electron transport chain. Because of its color change, the redox potential of the electron transport chain can easily be visualized with DCPIP.
DCPIP reduction can be halted by DCMU, which is a very effective herbicide. It blocks the plastoquinone binding site of photosystem II. Hence, it disables the whole electron transport chain.